EVER Pharma gains EU marketing authorization for value-added Cabazitaxel
EVER Pharma is pleased to announce EU-wide marketing authorization of Cabazitaxel for the treatment of adult patients with metastatic prostate cancer1.
The EVER product uses a novel formulation which allows it to be provided as a single vial and prepared to a final patient dose without any pre-dilution steps. This is a more convenient formulation that allows for the safe handling of this cytotoxic and saves time and costs compared to the currently available two vial system. The EVER product also adds two new presentations covering the common range of patient specific doses and reduces costly wastage by reducing the amount of left-over product after the patient dose has been extracted.
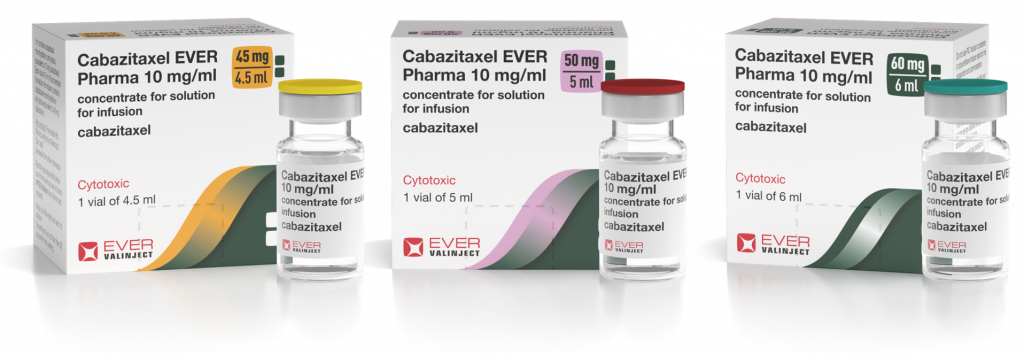
Use of Cabazitaxel is growing widely with the European market currently estimated to be worth more than €210m.
“Cabazitaxel is an expensive, yet critically important cancer treatment. We are pleased to be able to provide this innovative EVER product which enhances the safe and efficient preparation of the infusion and has the potential to meaningfully improve access to this therapy for the patient” said Georges Kahwati, General Manager of Ever Pharma.
The European approval of EVER Pharma Cabazitaxel follows its recent registration in Australia and subject to local patent restrictions will subsequently be launched in major markets around the world.
1 Cabazitaxel EVER Pharma in combination with prednisone or prednisolone is indicated for the treatment of adult patients with metastatic castration-resistant prostate cancer who have previously been treated with a docetaxel-containing regimen.
