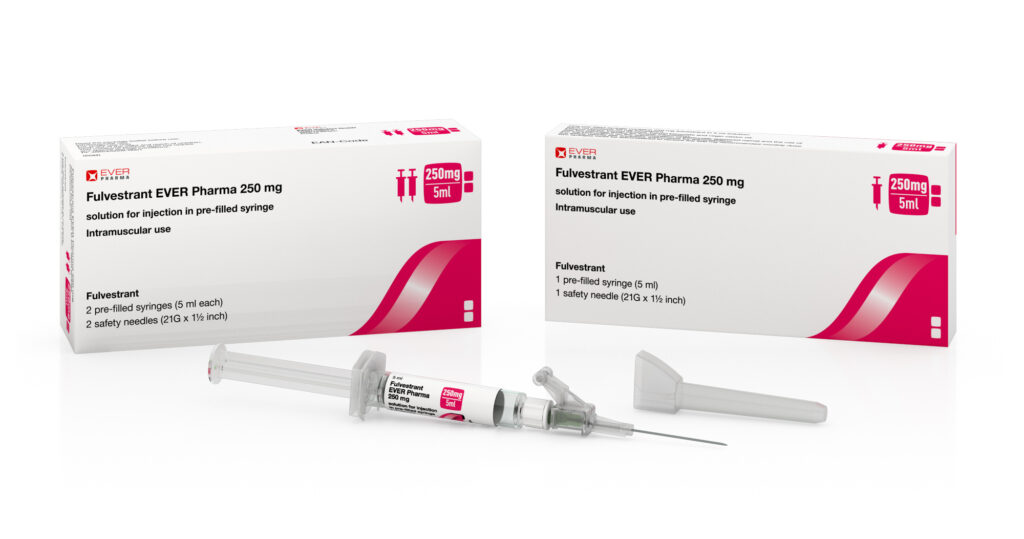
Fulvestrant
| Dosage Form | Solution for injection in pre-filled syringe |
| Strength | 250 mg |
| Strength per Unit | 250 mg/5 ml |
| ATC Code | L02BA03 |
| Drug Class | Endocrine therapy |
| Indication | Fulvestrant EVER Pharma in indicated: as monotherapy for the treatment of estrogen receptor positive, locally advanced or metastatic breast cancer in postmenopausal women: – not previously treated with endocrine therapy, or – with disease relapse on or after adjuvant antiestrogen therapy, or disease progression on antiestrogen therapy. in combination with palbociclib for the treatment of hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer in women who have received prior endocrine therapy (see section 5.1). In pre- or perimenopausal women, the combination treatment with palbociclib should be combined with a luteinizing hormone releasing hormone (LHRH) agonist. |
Fulvestrant EVER Pharma is a prescription medication. Information for healthcare professionals only.
See the Summary of Product Characteristics for full information about the medication.
